All those students who want to know all the important topics of the Dual Nature of Matter and Radiation chapter are reading the right article. In this article, we will discuss all the important topics that are related to the Dual Nature of Matter and Radiation chapter.
Class 12 Dual Nature of Matter and Radiation
The dual nature of radiation and matter
The minimum amount of energy required by an electron to just escape from the metal surface is called the work function of the metal.
Electron Volt
One electron volt is the kinetic energy gained by an electron when it is accelerated through a potential difference of 1 volt.
Energy gained by an electron = Work done by electric field = qV
1 eV = 1.602 x 10-19 J
The electron volt is a commonly used unit of energy in atomic and nuclear physics.
| Metal | Work Function (eV) |
| Cs | 2.14 |
| K | 2.30 |
| Na | 2.75 |
| Ca | 3.20 |
| Mo | 4.17 |
| Pb | 4.25 |
| Al | 4.28 |
| Hg | 4.49 |
| Cu | 4.65 |
| Ag | 4.70 |
| Ni | 5.15 |
| Pt | 5.65 |
Electron Emission
These phenomena of emission of electrons from a metal surface. For their release from the metal surface, electrons are supplied the required amount of energy by any one method:
- Thermionic emission
- Field emission or cold cathode emission
- Photoelectric emission
- Secondary emission
Dual nature of Light
In 1900, Planck proposed the quantization of EM waves and the existence of quanta of energy now called Photons.
Each photon has energy, E = hv
h: Planck’s constant
Where h = 6.626 x 10-34 J/s
= 4.136 x 10-15 eV/s
Properties of Photons
- Photon travels in a straight line with a speed c= 299,792,458 m/s in a vacuum. It is not a material particle but a packet of energy.
- The energy of a photon depends on the frequency and does not change with the medium.
E = hv = hc/λ
While changing medium speed and wavelength of photon changes the frequency remains constant.
- It is electrically neutral and is not affected by electric and magnetic fields.
- It does not exist at rest, its rest mass is zero.
Equivalent mass of the photon, E = mc2 = hv.
- The momentum of the photon:
P = mc = (h/λc) x c
P = h/λ
- The intensity of light is proportional to the number of photons in it.
The intensity of Light due to a Source
The energy crossing per unit area per unit time perpendicular to the direction of propagation is called the intensity of a wave.
Photon Flux
The number of photons incident on a normal surface per second per unit area.
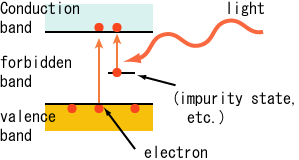
Photoelectric Effect
If the light of a suitable frequency illuminates a metal surface, electrons are emitted from the metal surface. The phenomenon is called the photoelectric effect. The photo (light)-generated electrons are called photoelectrons.
Laws of Photoelectric Emission
- For a given photosensitive material and frequency of incident radiation, (above the threshold frequency), the photoelectric current is directly proportional to the intensity of light. The saturation current is directly proportional to the intensity of incident radiation.
- The photoelectric emission is an instantaneous process. The time lag between the incidence of light radiation and the emission of photoelectrons is very small, even less than 10-9
- For a given photoelectric material, there exists a certain minimum cut-off frequency below which no photoelectrons are emitted, however high is the intensity of incident radiation. This frequency is called the threshold frequency.
- Above the threshold frequency, stopping potential or equivalently the maximum kinetic energy of the photoelectrons is directly proportional to the frequency of incident radiation, but is independent of its intensity.
Failure of classic Wave Theory
- According to the wave theory, when a wavefront of light strikes a metal surface, the free electrons at the surface absorb the radiant energy continuously. The greater the intensity of radiation, the greater is the amplitudes of electric and magnetic fields, and the greater is the energy density of the wave. Hence higher intensity should liberate photoelectrons with greater kinetic energy but this is contrary to the experimental result that the maximum kinetic energy of the photoelectrons does not depend on the intensity of incident radiation.
- No matter what the frequency of incident radiation, is a light wave of sufficient intensity should be able to impart enough energy required to eject the electrons from the metal surface.
Einstein’s Theory of Photoelectric Effect
The important points of Einstein’s theory of photoelectric effect:
- Photoelectric emission is the result of the interaction of two particles-one a photon of incident radiation and the other an electron of photosensitive metal.
- Each photon interacts with one electron. The energy hv of the incident photon is used up in two parts:
- A part of the energy of the photon is used in liberating the electron from the metal surface, which is equal to the work function Wo of the metal.
- The remaining energy of the photon is used in imparting the kinetic energy of the ejected electron.
- The free electrons are bound within the metal due to restraining forces on the surface. The minimum energy required to liberate an electron from the metal surface is called work function Wo of the metal.
- Very few (<1%) photons, whose energies are greater than Wo are capable of ejecting the photo-electrons.
By the conservation of energy,
The energy of the incident photon = Maximum K.E. of photoelectron + Work function
Kmax = ½ mvmax2 = hv-Wo
If the incident photon is of threshold frequency Vo then its energy hvo is just sufficient to free electrons from the metal surface and does not give it any kinetic energy. So hvo = Wo.
Kmax = ½ mvmax2 = hv-hvo
This equation is called Einstein’s photoelectric equations and can be used to explain the laws of photoelectric effect as follow:
- Explanation of the effect of intensity
- Explanation of threshold frequency
- A detailed explanation of time lag
Explanation of the effect of Intensity
The increase in intensity means the increase in the number of photons hitting the metal surface per unit of time. When Photon ejects only one electron, so the number of ejected photo-electrons increases with the increase in the intensity of incident radiation.
Explanation of threshold frequency
If v<v0 that is the frequency of incident radiation is less than the threshold frequency, the kinetic energy of photoelectrons becomes negative. This has no physical meaning. Hence, photoelectric emission does not occur below a threshold frequency.
Explanation of time lag
Photoelectric emission is the result of an elastic collision between a proton and an electron. Thus the absorption of energy from a photon by a free electron inside the metal is a single event that involves the transfer of energy in one lump instead of the continuous absorption of energy as in the wave theory of light. Hence there is no time lag between the incident of a photon and the emission of a photoelectron.
What is the Dual Nature of Matter and Radiation?
Particle Nature of Light: The Photon
When light interacts with matter, it behaves as if it is made up of discrete packets of energy, called quanta.
Some basic features of the photon picture of electromagnetic radiation are:
- When radiation interacts with matter, it behaves as if it is made up of particles, called photons.
- Photons are electrically neutral and are not deflected by electric and magnetic fields.
- Each photon has energy E = hv and momentum p = hv/c, and speed in a vacuum.
- All photons of light of a particular frequency v or wavelength λ, have the same energy E=hv=hc/λ and momentum p = hv/c = h/λ, independent of the intensity of the radiation.
Photo-Cell
Photo-Cell is an arrangement that converts light energy into electric energy. It works on the principle of photoelectric emission. It is also called a photoelectric cell.
Types of Photo-Cell
- Photo-emissive cell
- Photo-voltaic cell
- Photo-conductive cell
Applications of Photoelectric cells
- In photographic cameras
- Used in counting devices
- In cinematography
- In burglar’s alarm
Dual Nature of Matter
The materials particles in motion should display wave-like properties. His reasoning was based on the following two considerations:
- E=mc2 shows that there is a complete equivalent between matter (mass) and radiation (energy). There must be a mutual symmetry between matter and radiation.
- Natural loves Symmetry: Since radiations have a dual nature, therefore, from symmetry considerations, de-Broglie predicted that matter must also possess a dual nature. Thus particles like electrons, protons, neutrons, etc., should not only behave like a mass point but should also exhibit wave nature when in motion.
Thus waves associated with material particles in motion are called matter or de-Broglie waves and their wavelength is called de Broglie wavelength.
Wave Nature of Matter
The wavelength of matter waves associated with microscopic particles like an electron, proton, neutron, alpha particle, atom, molecule, etc., is of the order of 10-10 m, it is equal to the wavelength of X-rays, which is within the limit of measurement.
Properties of Matter Waves
- Matter waves are different from EM waves. They are related to moving particles and are independent of the fact whether the particle is neutral or charged.
- Matter waves travel at a speed more than that of light.
- Usually, λ is small for macroscopic objects and can be ignored.
- Wave and Particle aspects of moving bodies can never be observed at the same time. That is, the two are mutually exclusive.
- The Square of the amplitude of λ is proportional to the probability of finding the particle at that point.
Electron Microscope
It is an important application of de Broglie waves designed to study very minute objects like viruses, microbes, and the crystal structure of the solids. It was first designed by ERNST RUSKA in 1930.
Principle of Electron Microscope
- Like light radiation, electron beams behave as waves but with much smaller wavelengths.
- By using electric and magnetic fields, the electron beam can be focused just as ordinary light beams are focused on glass lenses.
Radiation Pressure
The force per unit area experience by the surface exposed to radiation is known as Radiation Pressure.
We hope You Like This Post “Dual Nature of Matter and Radiation”. Comment Below for other Physic Topic Class 12.
Helpful content
Very important notes
Very helpful
thanks, Ankit