Structure of the Atom for Class 9 Notes: In the article, discuss all important topics about the structure of the atom for class 9. Read this article and download the complete structure of the atom pdf notes for revision.
Contents
Definition of Atom
Atoms are made up of three subatomic particles such as electrons, protons and neutrons. Protons have a positive electrical charge, electrons have a negative electrical charge and neutrons have no electrical charge at all. Protons and neutrons are present in a small nucleus at the centre of the atoms. Electrons are outside the nucleus.
Atoms of all the elements (except hydrogen) are made up of three subatomic particles like electrons, protons and neutrons. A hydrogen atom is made up of just one electron and one proton. It does not contain any neutrons. The atoms of different elements differ in the no. of electrons, protons and neutrons.
Charged particles in matter
If we rub a glass rod with a piece of silk cloth, then the electrons from the glass rod are transferred to the silk cloth. Hence, the glass rod becomes positively charged. When the positive charge glass rod is brought near the inflated balloon, then the balloon will be attracted to the glass rod.
Similarly, if we rubbed a comb on dry hair, then this comb attracts small pieces of paper. The electric charge produced on the comb rubbing in hair comes from the atoms found in the comb.
SUBATOMIC PARTICLES OF ATOMS
- Electron
- Proton
- Neutron
DISCOVERY OF ELECTRON
The existence of electrons in an atom was discovered by J.J Thomson in 1897. When Thomson passed electricity at high voltage through gas at very low pressure taken in a discharge tube. A stream of minute particles was given out by the cathode (negative electrode). The steam of the cathode ray in the gas discharge tube consists of negatively charged particles called electrons. Atoms that contain negatively charged particles are called electrons.
- Electron is discovered by J.J. Thomson.
- The absolute mass of electron, Me = 9.1 x 10-31 kg
- The relative mass of the electron is 1/1840 u.
- The absolute charge of electron = – 1.6 x 10-19
- The relative charge of the electron is -1 (minus one).
- An electron is represented by e–.
DISCOVERY OF PROTON
The existence of protons in atoms was discovered by E. Goldstein / Rutherford.
When Goldstein passes at high voltage through gas at very low pressure taken in a discharge tube, streams of heavy particles were given out by the anode (positive electrode). These streams of particles are called anode rays. The anode rays obtained from hydrogen gas in the discharge tube consist of positive particles called protons.
The anode rays obtained from hydrogen gas in the discharge tube consist of positively charged particles called protons. It is formed by the removal of an electron from a hydrogen atom.
- The charge of a proton is equal and opposite to the charge of an electron.
- A proton is represented by the symbol p+.
- The absolute Charge of protons is 1.6 x 10-19
- The relative charge of a proton is +1 (plus one).
- The absolute mass of proton, Mp = 1.673 x 10-27 kg
- The relative mass of protons is 1 u.
- The mass of a proton is equal to the mass of a Hydrogen atom.
- Canal rays are positively charged radiation consisting of protons. Canal rays were discovered by Goldstein and this led to the discovery of the proton.
- Protons and neutrons found in the nucleus of an atom are together referred to as nucleons.
DISCOVERY OF NEUTRON
The existence of neutrons in atoms was discovered by James Chadwick in 1932. The neutron is a neutral particle present in the nucleus of an atom. Atoms of all the elements contain neutrons except hydrogen atom which does not contain any neutron. A neutron is a subatomic particle that is not found in hydrogen. A hydrogen atom contains only one proton and one neutron.
- The relative mass of a neutron is 1 u.
- The absolute mass of a neutron is 1.675 x 10-27
- A neutron is represented by the symbol n.
- Neutron has no charge.
- The neutron is located in the nucleus of the atom.
Three important models of the Structure of the atom
THOMSON’S MODEL OF THE ATOM
Thomson’s model of the atom is called the plum pudding model. The plum pudding model is one of the several historical scientific models of the atom. First proposed by J.J. Thomson in 1903 soon after the discovery of the electron but before the discovery of the atomic nucleus.
Thomson’s model of an atom is shown in the above figure. The coloured area in the watermelon contains all the positive charges in the atom. The negatively charged electrons are spread throughout the positive charge.
The total negative charge of electrons is equal to the total positive charge of the watermelon. These equal and opposite charges balance each other due to which an atom becomes electrically neutral on the whole.
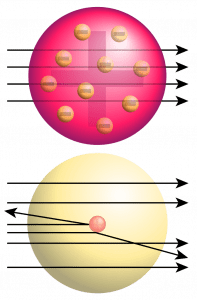
Gold foil is a very thin sheet of gold metal. Such a gold foil was used in Rutherford’s alpha particles scattering experiment which led to the discovery of the nucleus.
Rutherford’s Experiment – Discovery of Nucleus
When fast-moving alpha particles are allowed to strike a very thin gold foil in a vacuum, it is found that:
- Most of the alpha particles pass straight through the gold foil without any deflection from their original path; it shows that there is a lot of space in the atom.
- A few alpha particles are deflected through small angles and a few are deflected through large angles to show that there is a centre of positive charge in the atom which repels the positively charged alpha particles and deflects them to their original path. This centre of positive charge in the atom is known as the nucleus.
- The observation that a very few alpha particles completely rebound on hitting the gold foil and turn back on their path shows that the nucleus is very dense and hard which does not allow the alpha particles to pass through it.
Characteristics of the Nucleus
- The nucleus of an atom is positively charged.
- The nucleus is very dense and hard.
- It is very small compared to the size of the atom.
- The nucleus was discovered by Ernest Rutherford.
- The nucleus is a small positively charged part at the centre of an atom. It contains all the protons and neutrons.
- Protons and neutrons taken together are known as nucleons because they are present in the nucleus.
Rutherford’s model of the atom
Rutherford’s model is also called the nuclear atom or planetary model of the atom, a description of the structure of atoms proposed by Ernest Rutherford.
- An atom consists of a positively charged, dense and very small nucleus containing all the protons and neutrons. Almost the total mass of an atom is concentrated in the nucleus.
- Rutherford’s model proposed that the negatively charged electrons surround the nucleus of the atom. The electrons are revolving around the nucleus in circular paths at very high speeds. The circular paths of electrons are called orbits.
- The electron being negatively charged and the nucleus being a densely concentrated mass of positively charged particles are held together by the strong electrostatic force of attraction.
- An atom is electrically neutral because the number of protons and electrons in an atom is equal.
- Most of the atom is empty space.
- According to Rutherford’s theory, a hydrogen atom consists of a small nucleus containing one proton and one electron revolving around it. The nucleus is almost at the centre of the atom. Since the hydrogen atom contains an equal number of protons and electrons, it is electrically neutral. The nucleus of the ordinary hydrogen atom does not contain any neutrons.
Limitations of the Rutherford Atomic Model
- If we apply this electromagnetic theory to Rutherford’s atomic model, it will mean that the negatively charged particles electrons revolving around the nucleus with accelerated motion will lose their energy continuously by radiation. Thus, the energy revolving electrons will decrease gradually and their speed will also go on decreasing. The electrons will be attracted more strongly by the oppositely charged nucleus due to which they will come closer and closer to the nucleus and ultimately the electrons should fall into the nucleus by taking a spiral path. This should make the atom very unstable and hence the atom should collapse. But we know that atoms do not collapse on their own. Rutherford’s model does not explain the stability of an atom.
- One of the limitations of the Rutherford model was also that he did not say anything about the arrangement of electrons in the atom which made his theory incomplete.
- Rutherford’s model was unable to explain the stability of an atom. According to Rutherford’s model, electrons revolve at a very high speed around the nucleus of the atom in a fixed orbit. However, Maxwell explained that accelerated charged particles release electromagnetic radiation. Therefore, electrons revolving around the nucleus will release electromagnetic radiation.
Bohr’s Model of the Atom
A Danish physicist named Neil Bohr in 1913 proposed the Bohr atomic model. He modified the problems and drawbacks associated with Rutherford’s model of an atom. Bohr’s model of the atom can be described as follows:
- An atom is made up of three particles electrons, protons and neutrons. Electrons have a negative charge, protons have a positive charge and neutrons have no charge. Due to the presence of an equal number of negative electrons and positive protons, the atom, on the whole, is electrically neutral.
- The electrons revolve around the nucleus in fixed circular paths called energy levels or shells. The energy level is counted from the centre outwards.
- Each energy level is associated with a fixed amount of energy, with the shell nearest to the nucleus having minimum energy and the shell farthest from the nucleus having the maximum energy.
- The protons and neutrons are located in a small nucleus at the centre of the atom. Due to the presence of protons, the nucleus is positively charged.
- There is a limit to the number of electrons that each energy level can hold. The maximum number of electrons in energy level is 2n2, where ‘n’ is the number of energy levels. For example, the first energy level can hold a maximum of two electrons, the second energy level can hold a maximum of 8 electrons, the third energy level can hold a maximum of 18 electrons and the fourth energy level can hold a maximum of 32 electrons.
- There is no change in the energy of electrons as long as they keep revolving at the same energy level, and the atom remains stable.
Atomic Number
The number of protons in one atom of the element is known as the atomic number of the element.
- The atomic number is denoted by Z.
- Two elements cannot have the same element number.
- The atomic number of an element is equal to the number of electrons in a neutral atom of that element.
- All the atoms of the same element have the same number of protons in their nuclei, and hence they have the same atomic number.
| ELEMENT | SYMBOL | ATOMIC NUMBER |
| 1. Hydrogen | H | 1 |
| 2. Helium | He | 2 |
| 3. Lithium | Li | 3 |
| 4. Beryllium | Be | 4 |
| 5. Boron | B | 5 |
| 6. Carbon | C | 6 |
| 7. Nitrogen | N | 7 |
| 8. Oxygen | O | 8 |
| 9. Fluorine | F | 9 |
| 10. Neon | Ne | 10 |
| 11. Sodium | Na | 11 |
| 12. Magnesium | Mg | 12 |
| 13. Aluminium | Al | 13 |
| 14. Silicon | Si | 14 |
| 15. Phosphorus | P | 15 |
| 16. Sulphur | S | 16 |
| 17. Chlorine | Cl | 17 |
| 18. Argon | Ar | 18 |
| 19. Potassium | K | 19 |
| 20. Calcium | Ca | 20 |
Mass Number
The total number of protons and neutrons present in one atom of the element is known as its mass number. An atom consists of electrons, protons and neutrons. Since the mass of electrons is negligible, the real mass of an atom is determined by the protons and neutrons only.
- The mass number is also known as the atomic number.
- The mass number is denoted by A.
Mass number = No. of protons + No. of neutrons
OR
Mass number = Atomic number + No. of neutrons
| ELEMENT | SYMBOL | ATOMIC NUMBER | Mass Number |
| 1. Hydrogen | H | 1 | 1 |
| 2. Helium | He | 2 | 4 |
| 3. Lithium | Li | 3 | 7 |
| 4. Beryllium | Be | 4 | 9 |
| 5. Boron | B | 5 | 11 |
| 6. Carbon | C | 6 | 12 |
| 7. Nitrogen | N | 7 | 14 |
| 8. Oxygen | O | 8 | 16 |
| 9. Fluorine | F | 9 | 19 |
| 10. Neon | Ne | 10 | 20 |
| 11. Sodium | Na | 11 | 23 |
| 12. Magnesium | Mg | 12 | 24 |
| 13. Aluminium | Al | 13 | 27 |
| 14. Silicon | Si | 14 | 28 |
| 15. Phosphorus | P | 15 | 31 |
| 16. Sulphur | S | 16 | 32 |
| 17. Chlorine | Cl | 17 | 35.5 |
| 18. Argon | Ar | 18 | 40 |
| 19. Potassium | K | 19 | 39 |
| 20. Calcium | Ca | 20 | 40 |
Arrangement of Electrons in the atoms
Electrons are arranged to their potential energy in different energy levels or shells. The shells of the electrons are represented by the letters K, L, M, N, O, and P whereas energy levels are denoted by the numbers 1, 2, 3, 4, 5 and 6. The energy levels are represented by circles around the nucleus. K shell having minimum energy is nearest to the nucleus, L shell which has a little more energy is a bit farther away from the nucleus, and so on.
1st energy level is called the K shell.
2nd energy level is called the L shell.
3rd energy level is called the M shell.
4th energy level is called the N shell, and so on.
According to the Bohr-Bury scheme:
- The maximum number of electrons that can be accommodated in any energy level of the atom is given by 2n2.
The maximum number of electrons in the first energy level = 2n2
= 2 x (1)2
= 2
The maximum number of electrons in 2nd energy level = 2n2
= 2 x (2)2
= 8
The maximum number of electrons in the 3rd energy level = 2n2
= 2 x (3)2
= 18
The maximum number of electrons in the fourth energy level = 2n2
= 2 x (4)2
= 32
- The outermost shell of the atom cannot accommodate more than 8 electrons, even if it can accommodate more electrons.
- Electrons in an atom do not occupy a new shell unless all the inner shells are filled with electrons.
Electronic Configuration
The arrangement of electrons in the various shells of an atom of element is called electronic configuration. The maximum electrons which can be accommodated in the K shell are 2, for the L shell is 8, for the M shell is 18 and for the N shell is 32.
Valence Electrons
The electrons present in the outermost shell of an atom are called valence electrons. A valence electron is also called a valency electron.
Cause of Chemical Combination
The atoms combine to achieve the inert gas electron arrangement and become more stable. An atom can achieve the noble gas(or inert gas) electron arrangement in three ways:
- By losing one or more electrons
- By gaining one or more electrons
- By sharing one or more electrons
Valency of elements
The capacity of an atom of an element to form chemical bonds is known as its valency. It is decided by the number of valence electrons in its atom.
- The valency of a metal element is equal to the number of valence electrons in its atom.
- The valency of a non-metal element is usually equal to eight minus the number of valence electrons in its atom. There is one exception to this rule and that is the valency of hydrogen. The valency of hydrogen is equal to the number of valence electrons, which is 1 though hydrogen is a non-metal element.
Types of Valency
There are two types of valency:
- Electrovalency
- Covalency
Electrovalency
The number of electrons lost or gained by one atom of the element to achieve the nearest inert gas electron configuration is called its electrovalency.
Ex: Valency of magnesium – one magnesium atom loses 2 electrons to achieve the inert gas electron configuration, therefore, the valency of magnesium is 2(or +2).
Ex: Valency of oxygen – one atom of oxygen requires 2 electrons to achieve the nearest inert gas electron arrangement, so the electrovalency of oxygen is 2( or minus 2).
Covalency
The number of electrons shared by one atom of an element to achieve the nearest inert gas electron configuration is called covalency.
Example:
- Covalency of hydrogen à one atom of hydrogen shares 1 electron to achieve the nearest inert gas electron configuration, therefore, the covalency of hydrogen is 1.
- Covalency of nitrogen à one atom of nitrogen shares 3 electrons to achieve the nearest inert gas electronic configuration, therefore, the covalency of nitrogen is 3.
| ELEMENT | Electronic configuration | Valency |
| 1. Hydrogen | 1 | 1 |
| 2. Helium | 2 | 0 |
| 3. Lithium | 2,1 | 1 |
| 4. Beryllium | 2,2 | 2 |
| 5. Boron | 2,3 | 3 |
| 6. Carbon | 2,4 | 4 |
| 7. Nitrogen | 2,5 | 3 |
| 8. Oxygen | 2,6 | 2 |
| 9. Fluorine | 2,7 | 1 |
| 10. Neon | 2,8 | 0 |
| 11. Sodium | 2,8,1 | 1 |
| 12. Magnesium | 2,8,2 | 2 |
| 13. Aluminium | 2,8,3 | 3 |
| 14. Silicon | 2,8,4 | 4 |
| 15. Phosphorus | 2,8,5 | 3 |
| 16. Sulphur | 2,8,6 | 2 |
| 17. Chlorine | 2,8,7 | 1 |
| 18. Argon | 2,8,8 | 0 |
| 19. Potassium | 2,8,8,1 | 1 |
| 20. Calcium | 2,8,8,2 | 2 |
Different Between Isotopes and Isobars
Isotopes
Isotopes are atoms of the same element having the same atomic number but different mass numbers. Isotopes of an element have the same atomic number because they contain the same number of protons (and electrons). Isotopes of an element have different mass numbers because they contain different numbers of neutrons.
Example: 17Cl35 and 17Cl37 are the isotopes of chlorine.
Isobars
Isobars are atoms of different elements having different atomic numbers but the same mass number. Isobars have a different number of protons in their nuclei but the total number of nucleons in them is the same.
Example: 18Ar40 and 20Ca40 are an example of Isobars.
I hope you like this post “Structure of the atom”. Comment below for any suggestions related to this structure of the atom notes. Share these notes with your school friends.

This is a great blog.
Amazing article
I love reading your article content.
Hi my family member! I wish to say that this post is awesome, great written and include approximately all important infos. I would like to see more posts like this .
Excellent post. I was checking continuously this blog and I am impressed! Very useful information particularly the last part 🙂 I care for such info a lot. I was looking for this particular information for a very long time. Thank you and best of luck.